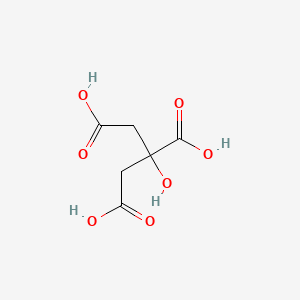
Citric Acid Ionic or Covalent
Increase your knowledge of the topics youll be tested on when you take the CLEP Biology exam with this study guide course. Chemical compounds are pure substances that are made up of two or more elements that are chemically combined in fixed mass ratios.
Are you a student of engineering and looking for the largest collection of engineering quiz questions for a practice test.

. The value of the pK a changes with temperature and can be understood qualitatively based on Le Châteliers principle. On the basis of the DFT calculations table S1 P-T generally has quadruple hydrogen bonds with interaction energy of 6836 kcalmol and the averaged interaction energy 1709 kcalmol is within the range of hydrogen bond of. The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction.
Is a proton H ion donor. Pure acids without water are composed of covalent small molecules. After that the mixture was heated at 30 C under string for 10 h and the dispersion of non-covalent modified RGO-CNTs was obtained.
Why submit to JCED. You collect a dried sample of the fungus and determine its heat of combustion to be -550 kJmol. Covalent compounds have weak force of attraction between their molecules so they are usually liquids or gases.
Which of the following statements about enzymes are true. Cells of the fungus have an ultimate analysis of mathrmCH_179 mathrmN_02 mathrmO_05 and the heat of formation of this species is necessary to approximate the heat duty for the bioreactor in which citric acid is to be produced. I a Ionic compounds have strong force of attraction between the oppositely charged ions eg Na and Cl so they are solids.
Is a substance that produces hydrogen ions H hydronium ions H 3 O when it dissolves in water Brønsted-Lowry theory. Group of answer choices. The pK a value is directly proportional to the standard Gibbs free energy change for the reaction.
You have arrived exactly at the right place. PH of Acids and Bases. What represents a formula for a chemical compound.
Theorys flaws is that it doesnt account for acid-base reactions that dont involve the development of a coordinate covalent bond. The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction. Choose all that apply.
Covalent bonding also includes several kinds of interactions such as σ-bonding π-bonding metal-to-metal bonding agostic interactions bent bonds three-center two-electron bonds 3c-2e and three-center four-electron bonds 3c-4e. Ionises completely in water to form a high concentration of H 3 O ions. JCED encourages manuscripts that report on consequential relevant comprehensive and robust data and place these data into context by addressing what can be learned from differences and similarities to prior published data on related systems.
After that the mixture was heated under 85 C and 100 ml citric acid was added. Therefore electrostatic interaction and hydrogen bonds played an important role in the loading of active herbicide in optimal-PCMs-SS carrier. As the term describes itself general engineering is the branch of science and technology that deals with many areas of science such as electrical mechanical chemical architectural civil and.
When water is added the pure acid molecules dissociate in water to form ions commonly referred to as the ionized acid Pure acids are poor conductors of electricity. A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F Enzymes lower. You can use the short.
160 ml NaSiO 3 and 5 ml ethanol were dripped into above dispersion of RGO-CNTs under room temperature. Moreover based on the polarity of the bond a covalent bond can be categorized as a polar covalent bond or a non-polar covalent bond. One of the hallmarks of JCED is that readers trust the results published in this.
The same type of interaction Lanthanum-modified chitosan oligosaccaharides Cos-La nanocontainers were prepared by simple ionic cross linking between oligosaccaharides and Lanthanum-citric acid complex. To find the numeric value of the acidity or basicity level of a substance the pH scale. Information for New Authors.
Acid Arrhenius theory. The capacity to explain the acidic or basic character of ionic species is one advantage of the Bronsted-Lowry definition of acids and bases. Examples of strong acids are hydrochloric acid HCℓ sulphuric acid H 2 SO 4 and nitric acid.
B Ionic compounds are soluble in water but covalent compounds are insoluble in water. Due to the mobile ions present acids diluted with water are considerably better conductors.

Comments
Post a Comment